Are you desperately looking for 'acid base chemistry essay'? Here, you will find all the stuff.
Acid-Base Chemistry ID Turn Chemical Name Chemic Formula 11A Carboxylic acid acid CH 3 COOH 11B Atomic number 1 nitrate HNO 3 11C Hydrogen sulphate H 2 Indeed 4 11D Element acid H 3 PO 4 August 29 2021
Table of contents
- Acid base chemistry essay in 2021
- Medical uses of acids and bases
- Application of base
- Importance of acids and bases
- Properties of acid with example
- What are bases
- Acid base lecture
- Acids and bases study guide
Acid base chemistry essay in 2021
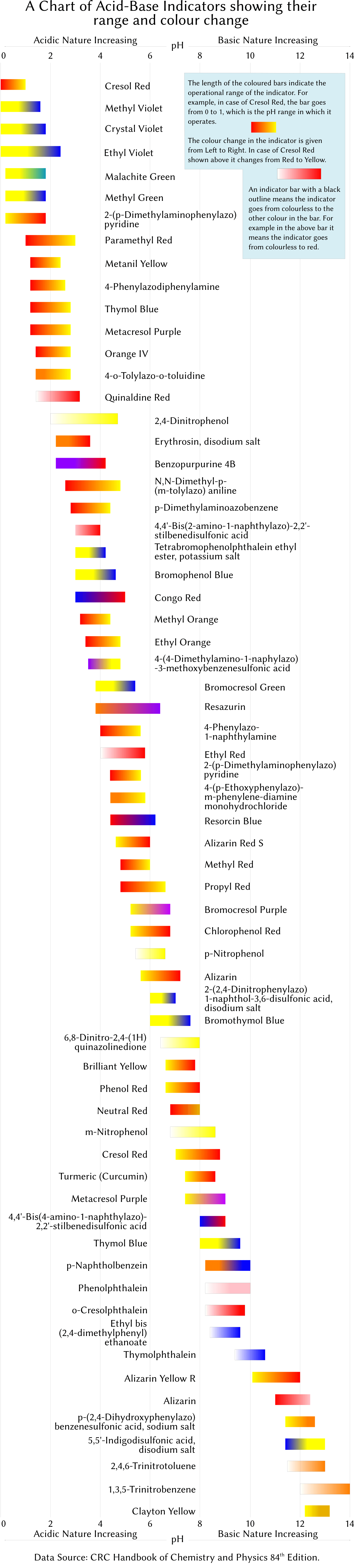 This picture shows acid base chemistry essay.
This picture shows acid base chemistry essay.
Medical uses of acids and bases
 This picture shows Medical uses of acids and bases.
This picture shows Medical uses of acids and bases.
Application of base
 This image representes Application of base.
This image representes Application of base.
Importance of acids and bases
 This image demonstrates Importance of acids and bases.
This image demonstrates Importance of acids and bases.
Properties of acid with example
 This picture illustrates Properties of acid with example.
This picture illustrates Properties of acid with example.
What are bases
 This picture representes What are bases.
This picture representes What are bases.
Acid base lecture
 This picture shows Acid base lecture.
This picture shows Acid base lecture.
Acids and bases study guide
 This image shows Acids and bases study guide.
This image shows Acids and bases study guide.
What is the conclusion of acid and bases?
Acids Acid And Bases Conclusion: Acids appear in products people use every day. To start, an acid is a compound that releases hydrogen ions in water. In order to find out if a substance is an acid, one must test it with litmus paper and a pH scale.
How are acids bases and buffers the same?
Acids Bases and Buffers – Essay Sample. An acid is defined as a proton donor. This is because when dissolved in water it dissociates releasing a proton (H +) which combines with water molecules to form an acidic solution [H 3 O +] > [OH –] on the other hand bases are defined as a substance present H3O + hence reducing their concentration.
Which is the product of an acid and a base?
When an acid dissociates or donates a proton the product is the conjugate base of that acid while a base ionizes to form the conjugate acid of that base these are termed as acid/ base pairs. Buffers are defined as solutions that resist PH airs.
How to find out if a substance is an acid?
Acids Acid And Bases Conclusion: Acids appear in products people use every day. To start, an acid is a compound that releases hydrogen ions in water. In order to find out if a substance is an acid, one must test it with litmus paper and a pH scale. On the pH scale acids measure from 1 to 6, pH 1 being the most acidic ...
Last Update: Oct 2021
Leave a reply
Comments
Aracelio
22.10.2021 11:11You could say this is an electrophile and then you could say Jerry Lee Lewis bases electron brace donor that's letter a nucleophile and nucleophile electrophile are exceedingly important concepts to understand when you're talking about constitutive chemistry.